As you may have heard from 2020 a new element will become visible on labels in the EU; a so called UFI code of 16 characters for all products classified for health or physical hazards. But what is this code about, why is it mandatory, what are the deadlines, who is responsible for registration, how to generate a UFI code and what about adjustments later on?
What is a UFI code?
UFI code, or fully pronounced ‘Unique Formula Identifier’ code, is a 16 character code which will be linked to a registration at poison centres and needs to be visible on product labels of products classified for health or physical hazards sold in the EU. This registration entails stating the formula of the product concerned, the product name, colour, type of packaging, product category and toxicological information.
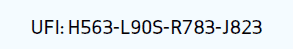
Why is it mandatory?
In case of an emergency all data of the particular product is right at hand. Poison centres have immediate access to all the specifications which can be a huge help to determine the approach to resolve the emergency and/or lower the risks and act fast.
Who is responsible for registration?
The company bringing the product on the market is responsible for registration. This means you are responsible for registration and adding the UFI code on the label for your own product(s). Keep in mind this registration is due 1st of January 2020 earliest (consumer products) and for products still on the market the deadline is 2025. As soon as there is a change in formula or label update the label must be adjusted and provided with a UFI code. We can add the UFI code as soon as there is a change in artwork.
It is also possible to outsource the creation of UFI codes. TecLub can generate a UFI code for the concerning product(s) and add this to the new label as soon as the label needs updating. Costs for changing the label are for your account.
What are the deadlines?
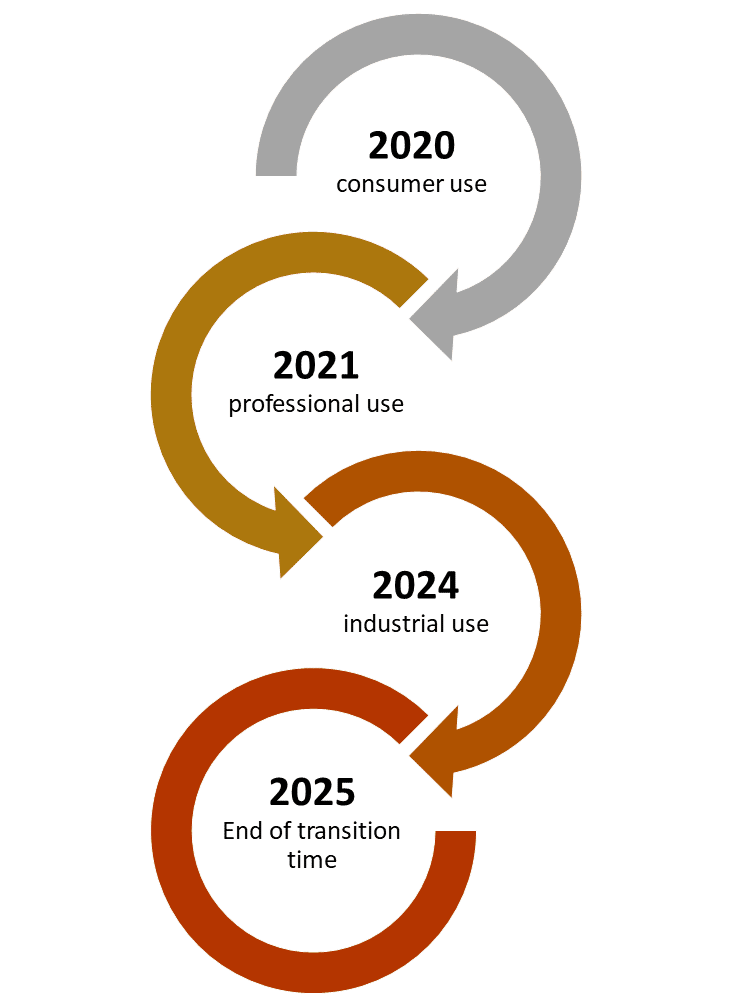
From the 1st of January 2020 it is obligatory to have a UFI code on all products classified for health or physical hazards meant for consumer use. Products meant for professional use must be registered by the 1st of January 2021. For industrial use the registering deadline is 1st of January 2024. For products which are already on the market and remain unchanged there is a transitional period until the 1st of January 2025. If the product needs to be updated before this date the new format must be implemented. However after that final date January 1st 2025 all products classified for health or physical hazards must be provided with a UFI code.
How to create a UFI code for registration?
For every country within the EU (28 member States and Norway, Iceland and Liechtenstein), where your product is on the market, you need to register in their language. The United Kingdom is still unclear at this moment due to its departure. For this registration you need to generate a UFI code for every product. How to create a UFI code depends on your choosing:
- TecLub generates a UFI code for you
To make this implementation easier TecLub can generate a UFI code for your product(s). Let you raccountmanager know you would like to outsource this or fill in our contact form. We will send you the UFI code(s) as soon as possible. You can register the UFI code(s) at poison centres in the countries concerned. - Generate the UFI code(s) at ECHA (European Chemical Agency) yourself at their website and register at the poison centers concerned.
Adjustment after UFI code generation
In case a UFI code is generated it is possible you have to make a new one every time:
- The formula has changed;
- Allowed variations of components change;
- The supplier changes the UFI code of one of the components (‘mixture in mixture’).

